Connect
to an infinite number of data sources seamlessly – from the newest, state-of-the-art systems to any legacy source.
to an infinite number of data sources seamlessly – from the newest, state-of-the-art systems to any legacy source.
your data to any protocol, industry standards and regulations with a library of shared, configurable data management rules and event-driven workflows.
data faster with automated acquisition and management tasks that seamlessly aggregate, transform, and validate your trial information.
your data using a comprehensive clinical review system that includes patient profiles with real-time alerts in a centralized location driven workflow.
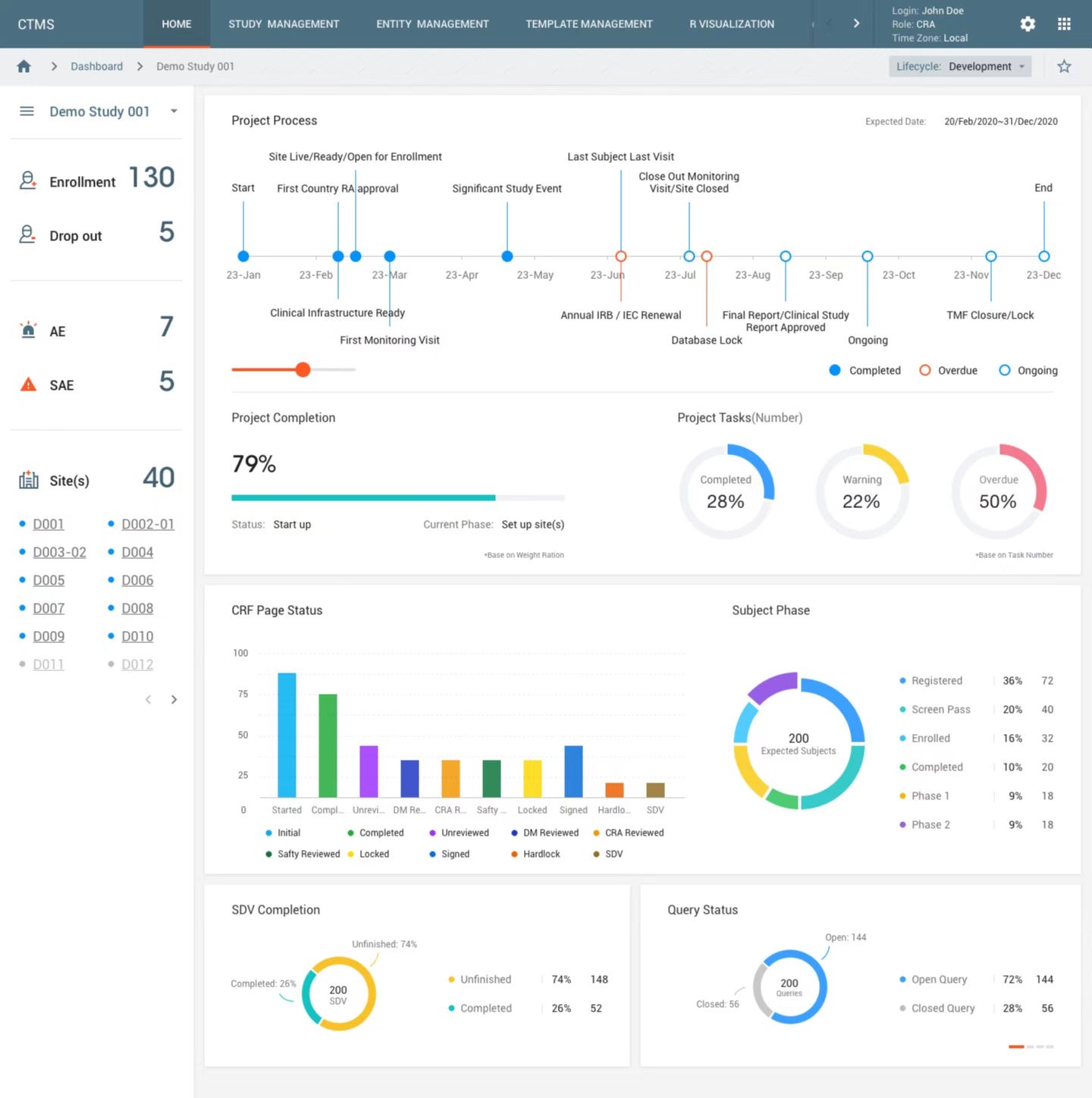
SIR enables the creation, setup and maintenance of studies in CONFORMTM. Study design components, data collection, processing and transformation workflows are defined and maintained throughout study execution and stored for reuse. Users can monitor study onset and execution across CONFORMTM and track progression of clinical activities conduct in near-real-time.
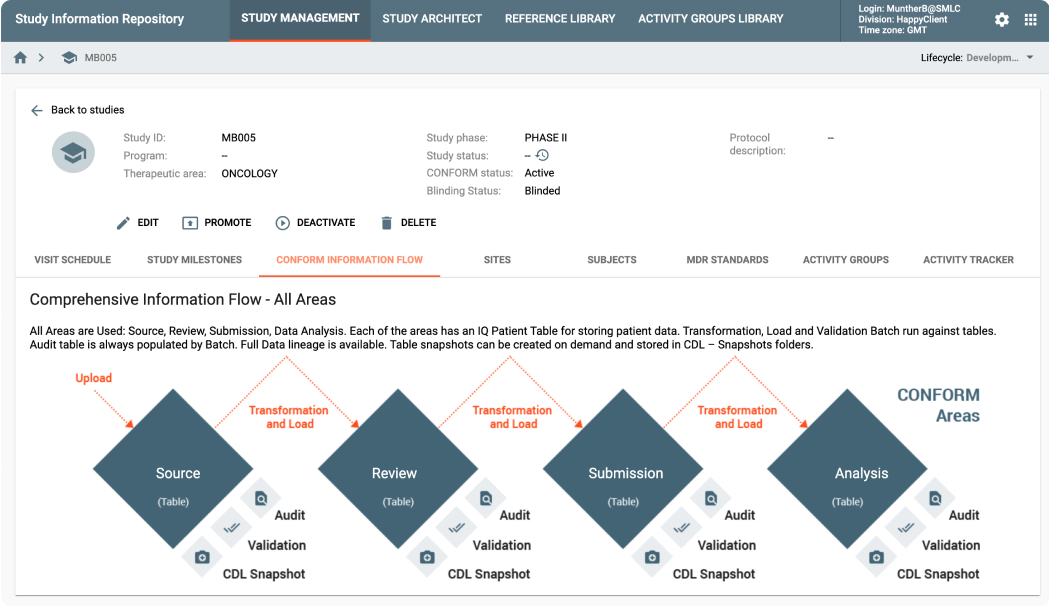
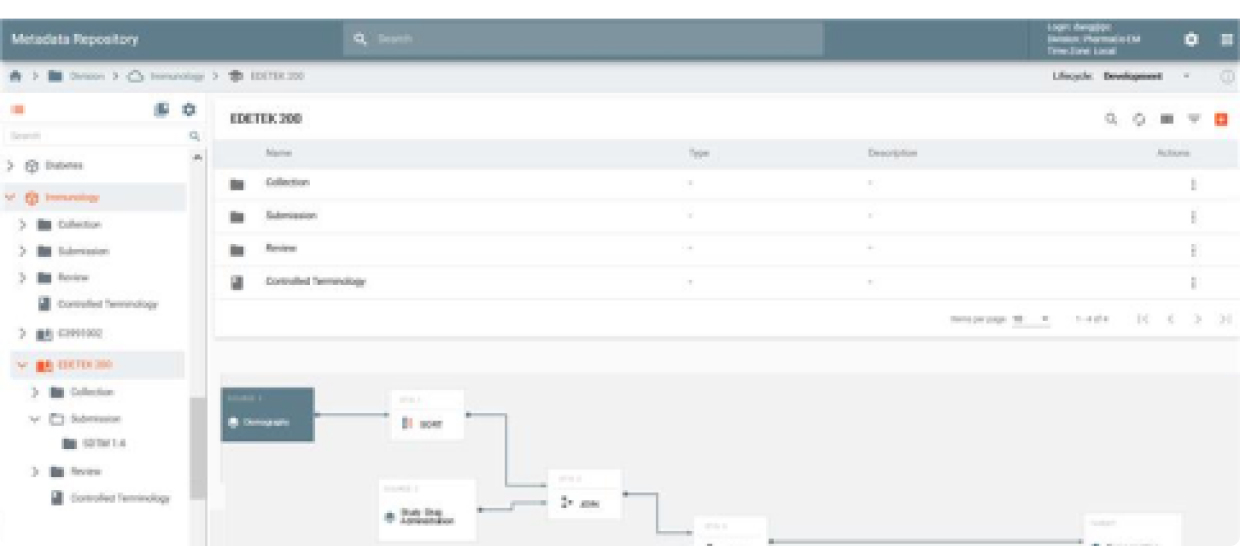
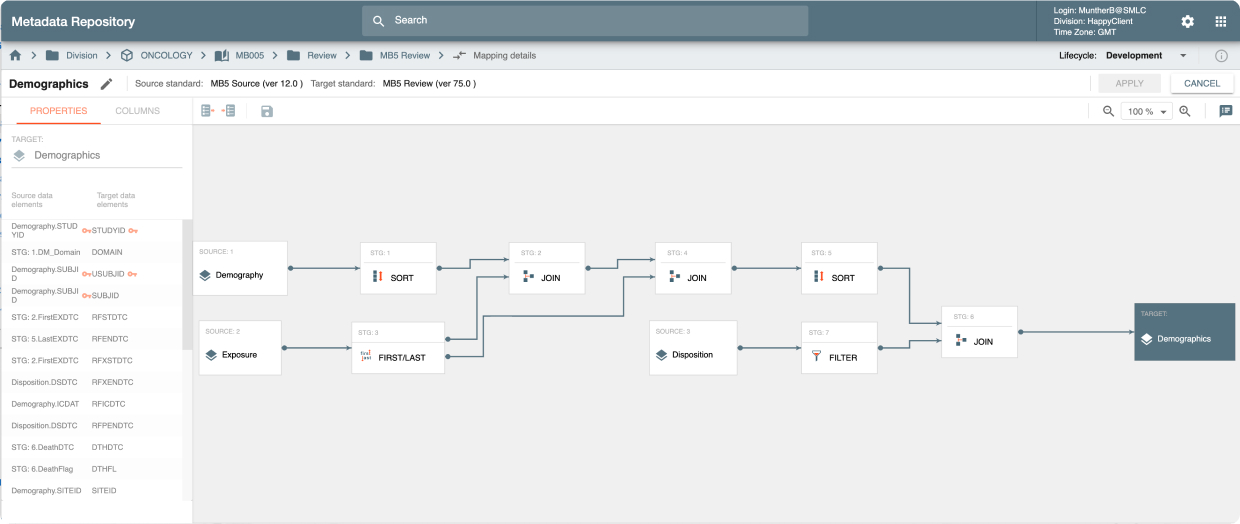
MDR is an API-driven repository that provides the ability to quickly setup study configurations using Industry, Company or Therapeutic Area standards. It also provides a powerful, graphically-driven capability to build Mappings and Transformations that allow data to be represented in standards supporting Data Review, Analysis, and Submission processes.
CDL is a global, secure and GxP compliant information repository that stores all structured and unstructured clinical data and documents. It maintains a configurable clinical hierarchy and an information store searchable by content, attributes, metadata and tags. CDL is a scalable and highly durable repository. It provides a Web interface to view content and to perform file management operations inside the Lake with all user actions automatically verified and logged.
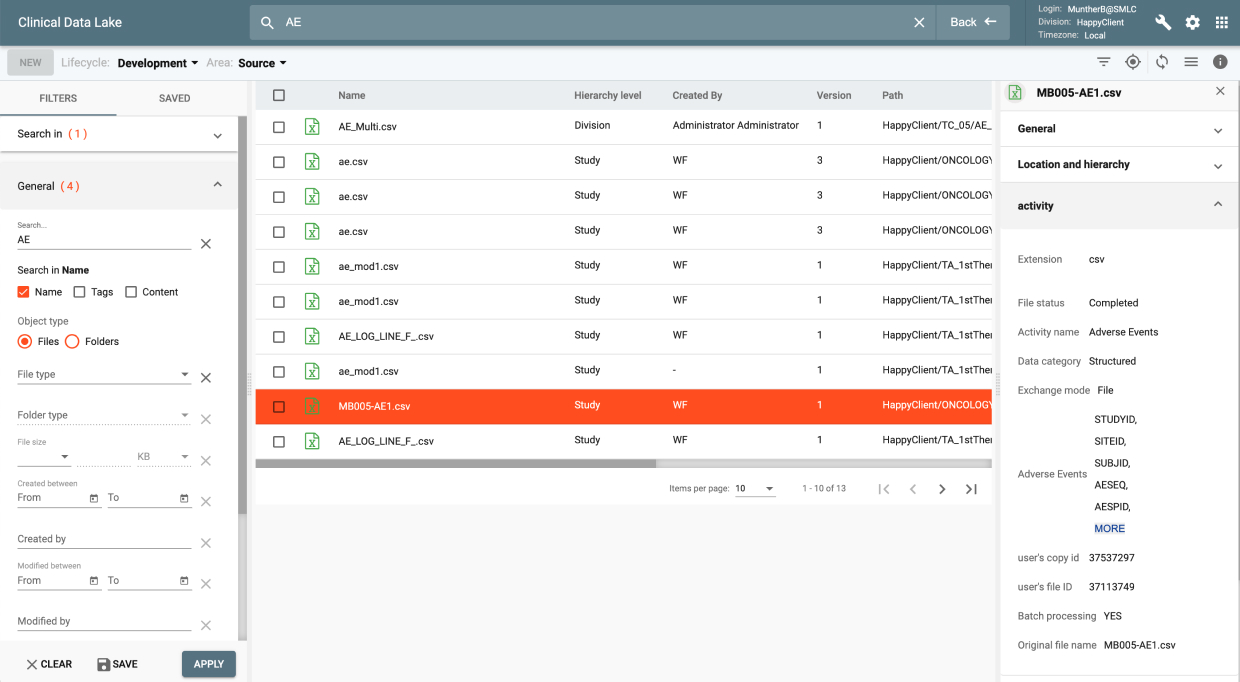
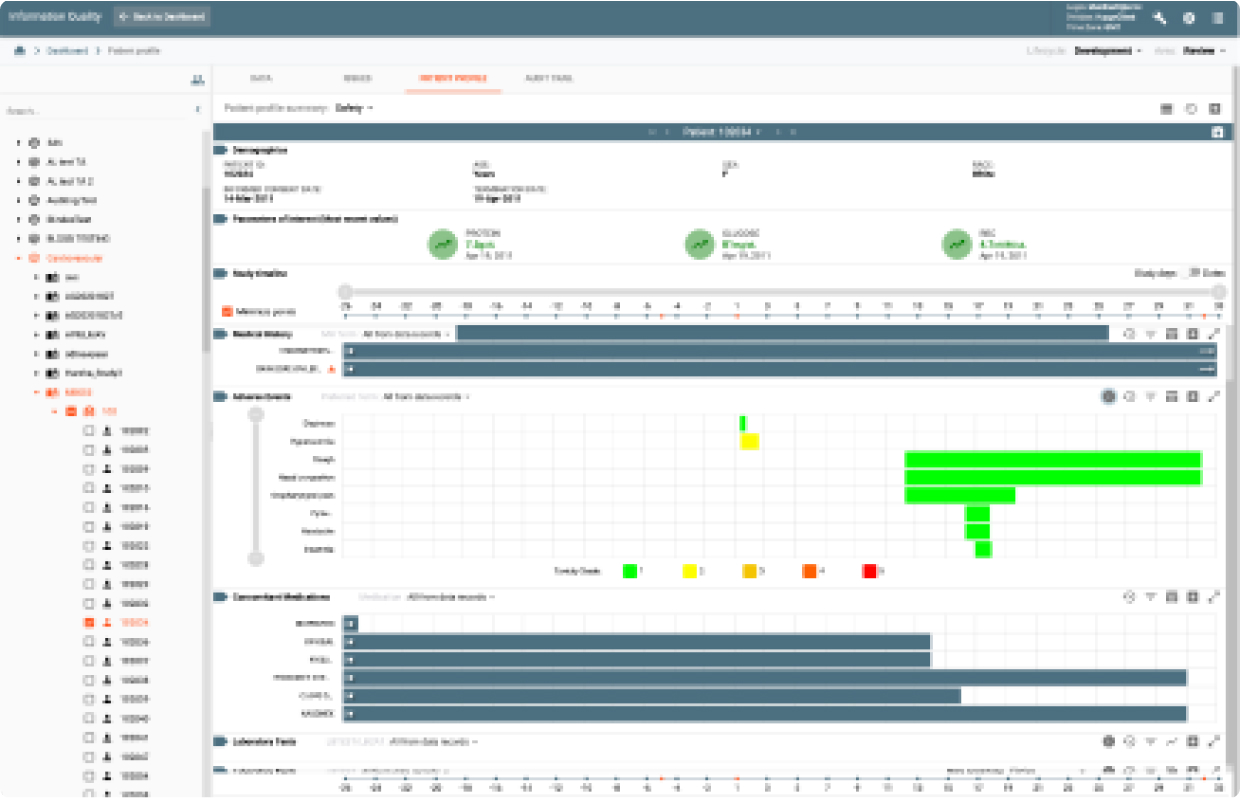
IQ provides data review capabilities for clinical data as it was collected from all data sources to its final target environment. It provides a complete audit trail and data lineage throughout all transformation stages. It also provides an integrated issue tracking system supporting Study, Site, and Patient level issues.
IQ’s Study Profiles display trends and stats with hot links to the underlying data while the Patient Profile Summary provides a longitudinal view of patient data for different data domains at the patient level over time for the data collected during the study.
Based upon medical, safety and efficacy business rules, alerts can be used to monitor clinical trial data in real-time. As changes occur in data, the alerts will fire and notify subscribers (e.g, scientists, data managers, site staff.)
DSP creates and maintains the SDTM/ADAM/TLF submission-ready packages.
DSP generates the full submission ready SDTM package by managing source datasets and eCRFs as well as solution level SDTM Standard, Controlled Terminology and Value Level Metadata; it prepares and generates annotated CRFs and transforms data to SDTM and related submission documents.
It is powered by AI/Machine Learning to perform CRF auto-SDTM Mapping, raw to standard SDTM transformation, and SDTM data validation.
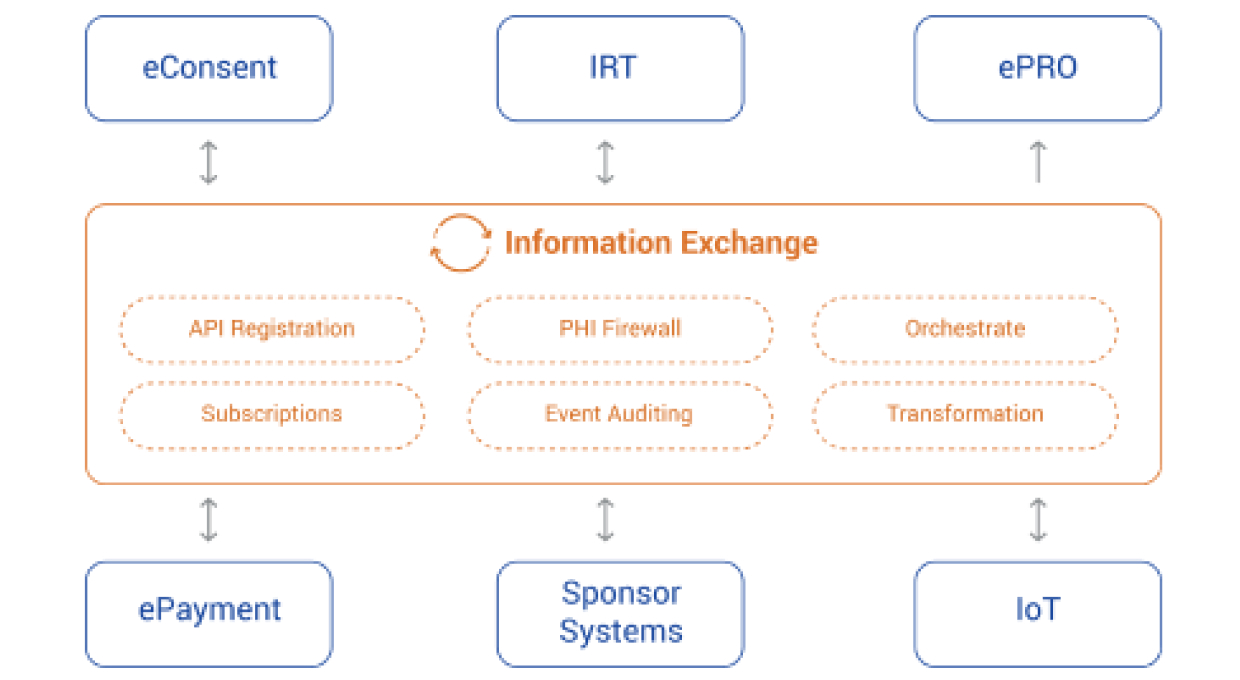
EMS records all business and scientific events across processes associated with systems and user activities within the platform. EMS Subscription service enables subscription-based actionable workflows and/or user notifications for configurable event types. EMS provides real-time orchestration of clinical events that occur during the execution of a clinical trial.
eClinical is a comprehensive universal portal consisting of Clinical Trial Management System (CTMS), Electronic Data Capture (EDC), Interactive Web Response System (IWRS), Trial Supply Management (TSM), ePayments, and electronic Trial Master File (eTMF). eClinical provides a complete solution for study design and conduct, data collection, safety monitoring, clinical data and documentation management, and many more.