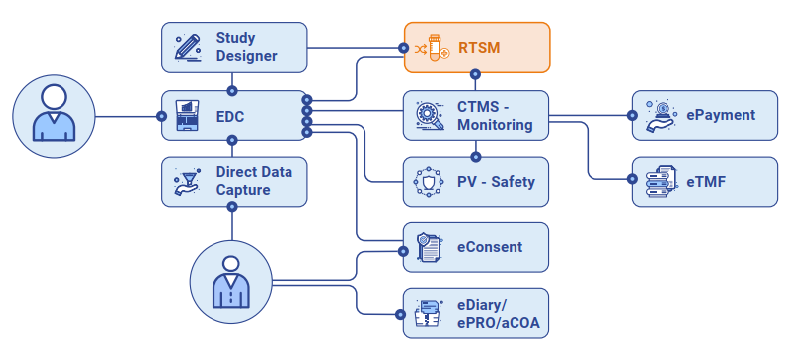
The eClinical Clinical Trial Management System (CTMS) is an advanced, integrated solution for managing the operational aspects of clinical trials. As part of the eClinical Platform, our CTMS ensures that clinical trial operations are optimized, streamlined, and compliant with industry standards—enhancing the efficiency and quality of your entire trial management process.
eClinical CTMS is uniquely designed to integrate seamlessly with other CONFORM™ modules, offering a fully unified platform for managing everything from data capture to patient engagement, compliance, and risk.
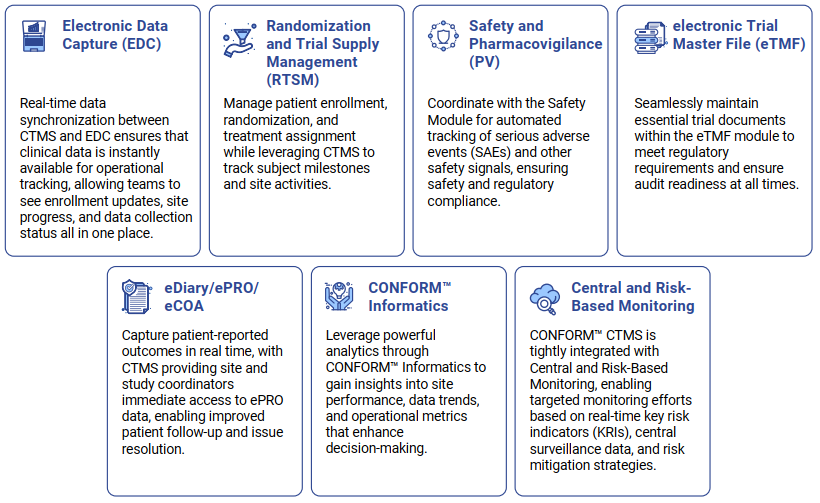
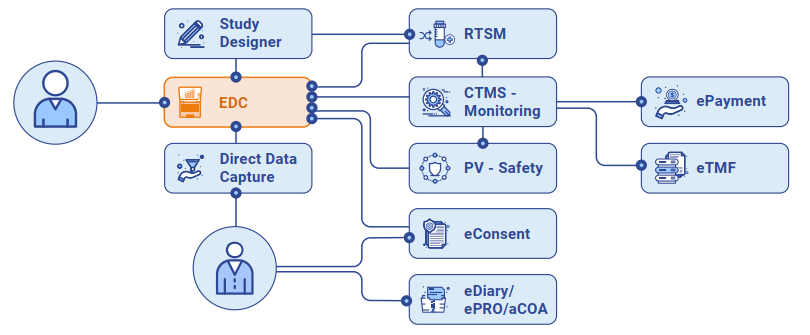
CONFORM™ EDC is a feature-rich data collection tool, fully integrated with the CONFORM™ Suite, providing for seamless trial execution.

The eClinical eDiary/ePRO/eCOA component is a comprehensive tool for managing patient-reported outcomes and other clinical assessments electronically. This module is an essential part of the eClinical Platform, providing a seamless experience for both patients and clinical staff, ensuring high-quality data capture, and enhancing patient engagement.
Designed to streamline clinical trials, eClinical eDiary/ePRO/eCOA enables the capture of patient-reported data and clinician assessments in real-time, offering a fully integrated, end-to-end solution that is interoperable with other CONFORM™ modules.
Capabilities include:


EDETEK presents its deep domain expertise with advanced technologies such as AI/ML, advanced analytics, and integrated data pipeline to launch a next generation RQBM solution. This RBQM solution offers a comprehensive, real-time approach to managing trial quality and monitoring risks, from data integration to issue resolution.
With advanced analytics and seamless integration with EDETEK’s innovative technology, you can trust our eClinical platform to deliver superior trial oversight.
The eClinical RTSM (Randomization and Trial Supply Management) is an essential part of the eClinical Platform, providing seamless randomization and trial supply management for clinical trials. As a part of an integrated, end-to-end solution, eClinical RTSM ensures efficient handling of patient assignments, treatment allocation, and investigational product management all from a single, intuitive platform.
Capabilities include: