Everyone wants to have a seamless data review and analytical system, but sometimes that can feel like an impossible mission. Perhaps you’ve tried countless Interactive data review and visualization tools that claim to create greater efficiency and productivity, reduce redundancy and cycle-time and improve decision-making, but nothing seems to work. This is especially challenging in complex trial designs coupled with variety of incoming data types.
That’s where our Information Quality (IQ) product comes in. We specifically designed our product from ground up to support ingestion of data from any clinical source in near-real-time to review and act on the data. Now you can finally be in control of your clinical trials as insights come to you rather you having to hunt for it.
Give Us a Try, Risk Free
Give us a try at no cost to you! We will work with you to configure your study, and you will have immediate access to review and analyze data and to assess quality. Don’t miss out on the future of data-driven clinical trials! Try our IQ product risk-free. Experience the difference yourself!
- Study data review tool responsible for the evaluation of data quality and clinical endpoints.
- Business-function configurable data review plan including critical data points and rule-based data cut off.
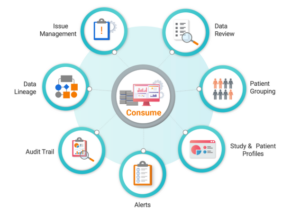
- Centralized issue management supporting study, site, and patient level issues.
- Summary screens integrated with the Study Navigator provides easy access to unreviewed records and records with open issues.
- Real time monitoring of significant medical observations such as optimal dose evaluation, DLT (Dose Limiting Toxicity), protocol banned CM, etc.
- Rich data visualizations for safety and efficacy data domains at the study and patient level over time.
- Full transparency of data transformations and derivations from source to target through the audit trail and data lineage capabilities.
- Many more….
What sets our product apart from the rest?
- Access: Timely access to data allows quicker intervention if the patient experiences safety concerns, including very early signals that may suggest or predict a challenge in the future.
- User Experience: Enhanced customer experience through personalized interactions, frictionless and seamless, and designed to achieve any function in 3 clicks or less.
- Analysis: CONFORM’s ability to process, analyze and assess clinical trial data in near-real time and in an automated fashion, streamlines review processes and leads to faster, data-driven decisions:
- Visual Clarity: Simplify and analyze complex data quickly.
- Spot Anomalies: Identify outliers and irregularities, improving error detection.
- Uncover Patterns: Reveal trends and correlations.
- Enhanced Collaboration: Present findings more effectively to stakeholders.
- Platform: CONFORMTM was designed from the ground up to support multiple clinical trial designs that use traditional and novel digital data collection systems and devices concurrently. It connects data producers and data consumers while storing data in one centralized, compliant location, making near real-time data transformation, validation, review, and analysis possible.
Transform your study information management data quality and analytics with CONFORMTM IQ. Try it today!
#ClinicalTrials, #DataAnalysis, #PatientSafety, #DataReview, #RealTimeMonitoring
 Unlock the true potential of data-driven decisions with our game-changing CONFORM Information Quality (IQ) product!
Unlock the true potential of data-driven decisions with our game-changing CONFORM Information Quality (IQ) product!




 back
back
 Previous
Previous